Pioneering the Future of Vaccines with Mucosal Immunity and Oral Delivery
At Vaxart, we are revolutionizing how the immune system responds and the way vaccines are delivered with our oral pill vaccine. Our diverse pipeline of prophylactic and therapeutic vaccines targets a range of infectious diseases. We are working to create a new generation of immunizations that are easier to administer, more accessible, and designed to meet the global health challenges now and in the future.
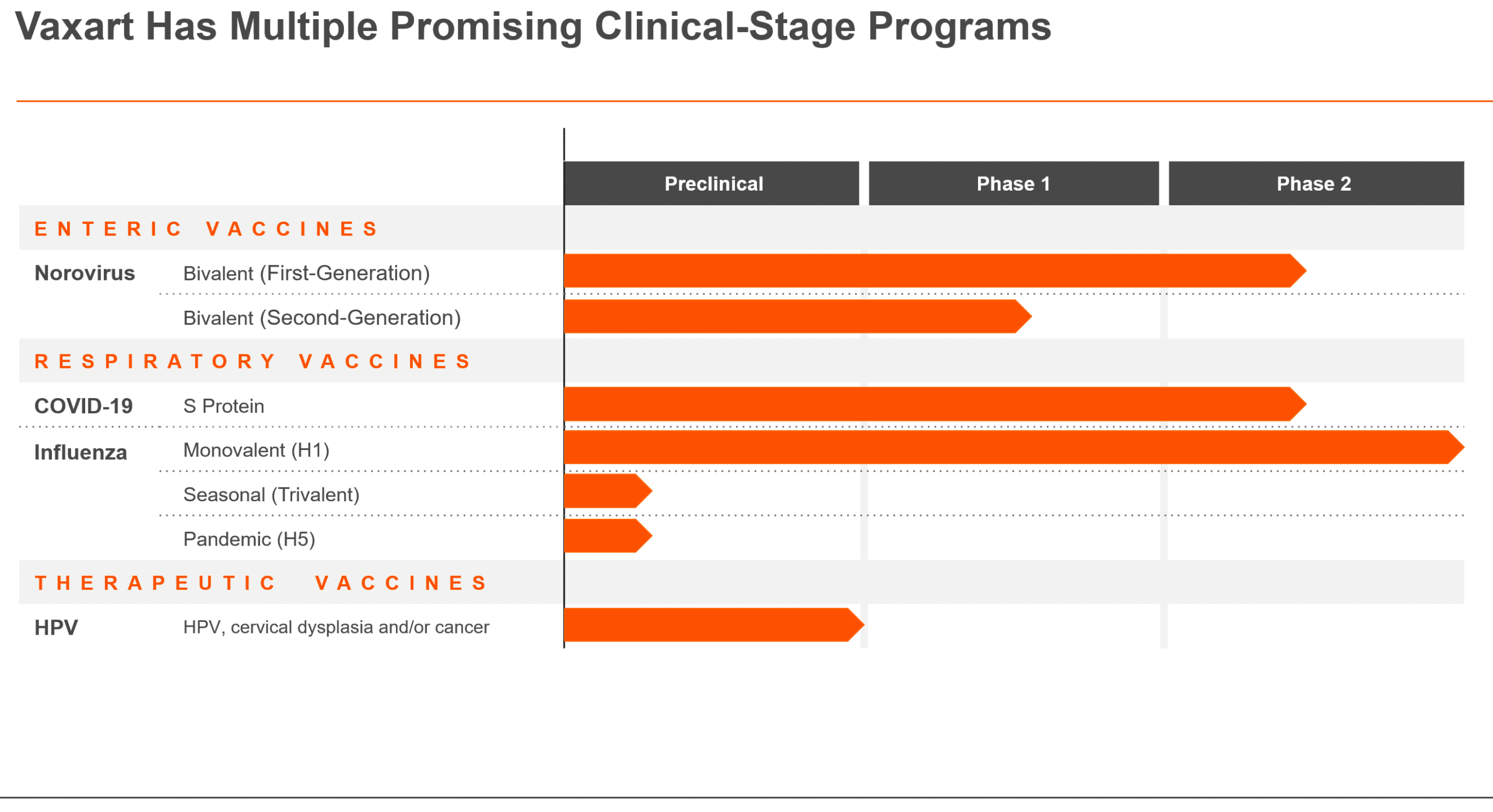
COVID-19
Innovating COVID-19 Vaccines for the Future: Vaxart’s Participation in BARDA’s Project NextGen
While existing COVID-19 vaccines remain effective at preventing severe illness and death, they are less capable of reducing infection and transmission over time. Emerging variants, waning immunity, and resistance to existing therapeutics highlight the ongoing need for innovative solutions to address these challenges.
Vaxart is collaborating with the Biomedical Advanced Research and Development Authority (BARDA) under Project NextGen.
Advancing COVID-19 Vaccine Research with BARDA’s Project NextGen
With an award from BARDA, Vaxart is focused on developing a novel oral pill vaccine that offers broader protection against SARS-CoV-2 variants, provides longer-lasting immunity, is easier to administer, and could help reduce transmission while enhancing global preparedness.
Phase 2b Trial
Vaxart is studying our oral pill vaccine candidate, VXA-CoV2-3.3, against an FDA-approved mRNA COVID-19 injectable booster vaccine in a 10,000 participant Phase 2b double-blind, multi-center, randomized, comparator-controlled clinical trial (NCT06672055).
The study’s goal is to determine the relative efficacy, safety, and immunogenicity of Vaxart’s oral pill COVID-19 vaccine candidate against an FDA approved injectable mRNA vaccine comparator in adults (≥18 years of age) previously immunized against COVID-19 infection.
The sentinel cohort comprised of 400 participants, with 200 receiving Vaxart’s oral pill COVID-19 vaccine candidate and 200 receiving an FDA-approved mRNA injectable vaccine comparator, has been fully enrolled.
As a pioneer of oral pill vaccines, Vaxart was the first U.S. company to complete a Phase 2 clinical trial of an oral pill vaccine for COVID-19.
BARDA Funding
Funding for this award was received under Project NextGen, a $5 billion initiative led by BARDA and the National Institute of Allergy and Infectious Diseases (NIAID) to accelerate and streamline the development of the next generation of innovative COVID-19 vaccines, therapeutics, and enablers.
Vaxart’s project award through the Rapid Response Partnership Vehicle (RRPV) is valued at up to $460 million. This project has been funded with federal funds from the U.S. Department of Health and Human Services (HHS); Administration for Strategic Preparedness and Response (ASPR); BARDA, under Other Transaction (OT) number 75A50123D00005.
Previous Clinical Trial Results
Vaxart’s Phase 1 clinical trials demonstrated similar oral pill vaccine candidates triggered robust mucosal and T-cell responses, which should allow the body to fight infection where it invades— in the mucosa.
Pre-clinical research also suggests that Vaxart’s previous oral pill COVID-19 vaccine candidate inhibits airborne transmission of the virus by reducing shedding. These results are consistent with those from Vaxart’s Phase 2 human influenza challenge study, which showed Vaxart’s oral pill vaccine was better at reducing shedding than the injectable flu vaccine comparator. The vaccine has shown cross-reactivity against many of the evolving variants of the current COVID-19, including previous highly pathogenic outbreak strains such as the initial SARS and MERS.
COVID-19 Disease Burden
Despite the availability of vaccines and therapeutics, COVID-19 remains a significant public health concern.
| Cumulative Disease Burden (2024) |  Worldwide Worldwide |  US US |
|---|---|---|
 Infections Infections | 750 M | 100 M |
 Deaths Deaths | 7.1 M | 1.2 M |
Source: World Health Organization, WHO COVID-19 Dashboard
Vaxart’s Oral COVID-19 Pill Vaccine
A new generation of vaccines is needed to address the limitations of currently FDA-approved mRNA vaccines. Vaxart’s orally delivered vaccine holds significant promise to meet the evolving challenges posed by new variants and waning immunity in an easy-to-administer pill while potentially offering broad protection.
Norovirus
Vaxart’s Novel Approach: an Oral Norovirus Pill Vaccine Designed to Provide Mucosal Immunity and Protect Infants, Adults, and Seniors
Norovirus is a leading cause of acute gastroenteritis (AGE) globally, responsible for nearly 20% of all episodes of diarrheal disease. Currently, there are no approved vaccines available to prevent norovirus.
Vaxart is developing a bivalent oral pill vaccine targeting the two genogroups most associated with norovirus infections in people. With the potential for cross-protection, clinical trials in adults and seniors have demonstrated strong immune responses, supporting its promise as a convenient, needle-free vaccine option.
Vaxart received funding and support from the Gates Foundation to investigate whether immunizing nursing mothers with its oral pill norovirus vaccine candidate produced antibodies that could protect their breastfeeding infants.
Norovirus
Vaxart’s Oral Pill Vaccine Targets Norovirus with Mucosal Immunity
Vaxart is developing a bivalent, oral pill vaccine for norovirus. Because norovirus is a pathogen that infects the small intestine, Vaxart believes a vaccine that produces mucosal antibodies locally in the intestine, in addition to systemic antibodies that circulate in the blood, may better protect against norovirus infection than an injectable vaccine.
Norovirus – A Widespread Risk For Everyone
Norovirus is highly contagious and spreads very easily and quickly. Anyone can contract norovirus, with most people experiencing at least one infection by age 5 and up to 8 episodes in their lifetime.
While most people infected with norovirus recover in a few days, children under the age of five years, older adults and individuals with compromised immune systems can experience more severe disease.
Importantly, due to its high transmissibility, short incubation period, and lack of symptoms in 30% of infections, norovirus can spread rapidly, especially in closed or semi-closed settings such as dormitories, daycare centers, long-term care facilities, hospitals, and cruise ships.
The lack of an approved norovirus vaccine is a significant public health challenge.
| Annual Disease Burden |  Worldwide Worldwide |  US US |
|---|---|---|
 Infections Infections | 685 M | 20 M |
 Deaths and Hospitalizations Deaths and Hospitalizations | 200K Deaths (50k among children) | 900 Deaths (mostly older adults) 100K Hospitalizations |
 Economic Costs Economic Costs | $60 BN | $10 BN |
Source: Carlson, K.B., Dilley, A., O’Grady, T. et al. A narrative review of norovirus epidemiology, biology, and challenges to vaccine development. npj Vaccines 9, 94 (2024). https://doi.org/10.1038/s41541-024-00884-2
Gates Foundation Sponsored Study
A Phase 1 study in lactating mothers showed that our oral pill vaccine candidate resulted in a 4-6-fold increase in norovirus antibodies in breast milk, which may help to protect infants through passive antibody transfer.
Phase 2 Challenge Study
In a Phase 2 challenge study, our oral norovirus pill vaccine candidate produced a statistically significant reduction in infection rate, a non-statistically significant reduction in norovirus AGE, and a substantial reduction in viral shedding.
Influenza
Vaxart’s Oral Pill Vaccine: A Promising Alternative to Traditional Flu Shots
The unmet medical need for influenza prevention remains significant despite the availability of seasonal flu vaccines. Current flu vaccines offer variable protection with effectiveness ranging from 40% to 60% depending on the season and how well the vaccine matches circulating strains.
In a completed Phase 2 study, the efficacy of Vaxart’s oral pill vaccine compared favorably to market-leading Fluzone®.
Influenza Continues to be a Serious Public Health Issue
Flu is a contagious respiratory illness caused by influenza viruses that infect the nose, throat, and sometimes the lungs. Anyone can get the flu, but certain groups are at higher risk of developing severe complications and even death. These include young children, older adults, pregnant women, and people with chronic health conditions like asthma, diabetes, or heart disease. Additionally, individuals with a body mass index (BMI) of 40 or higher are also at increased risk.
Influenza is a major cause of morbidity and mortality worldwide and in the United States. The flu virus frequently mutates, leading to antigenic drift, which can render vaccines less effective against emerging strains.
| Annual Disease Burden |  Worldwide Worldwide |  US US |
|---|---|---|
 Infections Infections | ~1 BN | 9.3 M – 41 M |
 Deaths and Hospitalizations Deaths and Hospitalizations | 290 K to 650 K Deaths | ~5K – 51K Deaths 100K – 710K Hospitalizations |
Sources: World Health Organization. News Room / Fact Sheet / Detail / Influenza (Seasonal) (3 October 2023).
CDC. Flu Burden. (2 August 2024).
Phase 2 Challenge Study Demonstrated Comparable Efficacy to Market Leader
The results of Vaxart’s Phase 2 randomized, double-blind clinical trial (NCT02918006) for its H1 influenza oral pill vaccine were published in The Lancet Infectious Diseases on January 21, 2020. The study demonstrated that a single dose of our oral pill vaccine was well-tolerated and provided statistically significant protection against H1 influenza in a human challenge model.
When compared to Fluzone®, an injectable quadrivalent influenza vaccine (QIV), the oral pill vaccine showed similar efficacy:
- 39% reduction in clinical disease, compared to 27% with Fluzone.
- 47% reduction in infection rates, compared to 43% with Fluzone.
The study also showed that our oral pill vaccine primarily works through mucosal immunity, which may be a key factor in improving flu immunization performance. The vaccine had a safety profile similar to placebo.
Vaxart’s Oral Pill Vaccine for Public Health
Our oral pill influenza vaccine could revolutionize flu immunization by offering a more accessible, effective, and convenient alternative to current injectable vaccines. Our oral pill vaccine could enhance flu prevention and contribute to broader public health efforts. A wider acceptance of an oral pill vaccine could increase vaccination rates. A mucosal and systemic immune response could potentially reduce flu transmission, ultimately saving lives and reducing healthcare costs.
Human Papilloma Viruses (HPV)
Vaxart’s HPV Therapeutic Vaccine: A Promising Non-Invasive Treatment for Cervical Pre-cancers
Human papillomavirus (HPV) is a common virus that can cause cancers later in life. HPV 16 and HPV 18 are responsible for approximately 70% of cervical cancers and 50% of high-grade pre-cancerous cervical lesions.
Vaxart’s HPV therapeutic vaccine shows promise as a novel, non-invasive treatment for HPV-related cervical dysplasia.
Cervical Cancer is the Fourth Most Common Cancer in Women Globally
Human papillomavirus (HPV) is a widespread sexually transmitted infection that can affect the skin, genital area, and throat. Approximately 85% of all sexually active individuals will be infected at some point in their lives, often without showing symptoms.
In most cases, the immune system clears the virus from the body naturally. However, persistent infection with high-risk strains of HPV can lead to the development of abnormal cells, which, if left untreated, may progress to cancer.
| Annual Disease Burden (2024) |  Worldwide Worldwide |  US US |
|---|---|---|
 Cervical Cancer Cervical Cancer | 600 K new cases | 11 K Cervical cancer 196 K Cervical pre-cancers |
 Deaths Deaths | 350 K 2022 | 4 K |
Sources: World Health Organization. News Room / Fact Sheets / Detail / Cervical Cancer (5 March 2024).
CDC. Human Papilloma Virus (HPV). Clinical Overview of HPV. (9 July 2024).
A Step Towards Global Equity in HPV-related Cancer Treatment
At Vaxart, we are developing an oral pill HPV therapeutic vaccine that offers a non-invasive treatment option for HPV-related cervical dysplasia. Preclinical studies have shown that our vaccine stimulates specific T-cell immune responses, reduces tumor size, and increases survival in animal models of HPV-related cancer. Our vaccine candidates target HPV proteins responsible for transforming healthy cells into malignant ones, prompting a potent immune response.
Our innovative mucosal platform holds promise for a novel, non-invasive treatment approach that could clear persistent HPV infections and promote regression of precancerous lesions. Furthermore, since our vaccine can be administered orally and is stable at room temperature, it has the potential to address global healthcare disparities, particularly in low-resource settings, where access to treatments may be limited.

Publications
COVID-19
Vaccines
January 2024
Frontiers Immunology
February 2020
Science Translational Medicine
May 2022
The Journal of Infectious Diseases
November 2021
Nature Medicine
January 2021
Norovirus
Science Translational Medicine
March 2025
Journal of Clinical Investigation
July 2018
Influenza
Cell Host and Microbe
December 2021
The Lancet Infectious Disease
April 2020
The Lancet Infectious Disease
September 2015
Nature Scientific Reports
November 2016
Human Papilloma Virus
The Platform
Vaccines
October 2023
Vaccines
April 2022
Expert Review of Vaccines
2008