Pioneering the Future of Vaccines with
Mucosal Immunity and Oral Delivery
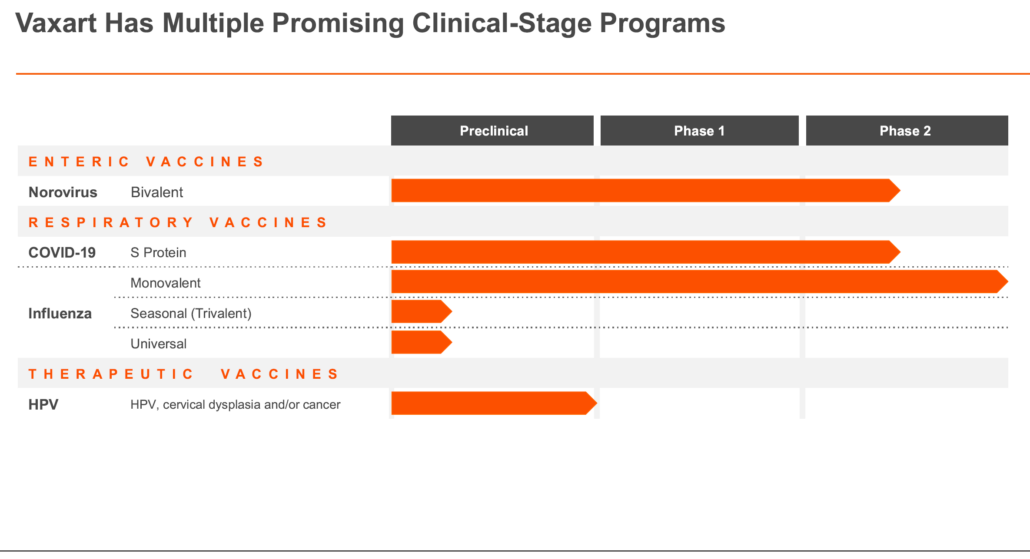
At Vaxart, we are revolutionizing the way vaccines are delivered and how the immune system responds. By harnessing the power of our pill, our diverse pipeline of prophylactic and therapeutic vaccines targets a wide range of infectious diseases, creating a new generation of vaccines that are easier to administer, more accessible, and designed to meet global health challenges now and in the future.
COVID-19
Innovating COVID-19 Vaccines for the Future: Vaxart’s Partnership with BARDA’s Project NextGen
While existing COVID-19 vaccines remain effective at preventing severe illness and death, they are less capable of reducing infection and transmission over time. Emerging variants, waning immunity, and resistance to existing therapeutics highlight the ongoing need for innovative solutionst o address these challenges.
Vaxart is collaborating with the Biomedical Advanced Research and Development Authority (BARDA) under Project NextGen.
Advancing COVID-19 Vaccine Research with BARDA’s NextGen Program
Vaxart’s partnership with BARDA focuses on developing a novel vaccine that offers broader protection against SARS-CoV-2 variants, provides longer-lasting immunity, is easier to administer and could help reduce transmission while enhancing global preparedness
Phase 2b Trial
Vaxart is studying its oral pill vaccine candidate, VXA-CoV2-3.1, against a currently approved mRNA COVID-19 injectable booster vaccine in a 10,000 participant Phase 2b double-blind, multi-center, randomized, comparator-controlled clinical trial (NCT06672055).
The study’s goal is to determine the relative efficacy, safety, and immunogenicity of Vaxart’s oral pill COVID-19 vaccine candidate against Pfizer’s injectable COMIRNATY® in adults (≥18 years of age) previously immunized against COVID-19 infection.
Sentinel Cohort
The sentinel cohort (NCT05067933) comprised of 400 participants, with 200 receiving Vaxart’s COVID-19 vaccine candidate and 200 receiving an approved mRNA vaccine comparator, has been completed.
As a pioneer of oral vaccines, Vaxart was the first U.S. company to complete a Phase 2 clinical trial of an oral vaccine for COVID-19.
BARDA Funding
Funding for this award was received under Project NextGen, a $5 billion initiative led by BARDA and the National Institute of Allergy and Infectious Diseases (NIAID) to accelerate and streamline the development of the next generation of innovative COVID-19 vaccines, therapeutics, and enablers.
Vaxart’s project award through the Rapid Response Partnership Vehicle (RRPV) is valued at up to $460 million. This project has been funded with federal funds from the U.S. Department of Health and Human Services (HHS); Administration for Strategic Preparedness and Response (ASPR); BARDA, under Other Transaction (OT) number 75A50123D00005.
Previous Clinical Trial Results
Vaxart’s Phase I clinical trials demonstrated its vaccine candidate triggers robust mucosal and T-cell responses, which should allow the body to fight infection where it invades— in the mucosa.
Pre-clinical research also suggests that Vaxart’s Covid-19 vaccine candidate inhibits airborne transmission of the virus by reducing shedding. These results are consistent with those from Vaxart’s Phase II human flu challenge study, which showed Vaxart’s oral tablet vaccine was better at reducing shedding than the injectable flu vaccine comparator. The vaccine has shown cross reactivity against many of the evolving variants of the current COVID-19, including Delta and older versions such as the initial SARS and MERS.
COVID-19 Disease Burden
Despite the availability of vaccine and therapeutics, COVID-19 remains a significant public health concern.
| Cumulative Disease Burden | ||
|---|---|---|
| Infections | 750 M | 100 M |
| Deaths | 7.1 M | 1.2 M |
The Promise of Vaxart’s Oral Pill Vaccine
A new generation of vaccines is needed to address the limitations of current mRNA vaccines. Vaxart’s orally administered vaccine holds significant promise to meet the evolving challenges posed by new variants and waning immunity in an easy-to-administer pill while offering broad protection.
Norovirus
Vaxart’s Novel Approach: Protecting Breastfeeding Infants by Vaccinating Lactating Mothers with
an Oral Norovirus Vaccine
Norovirusis a leading cause of acute gastroenteritis (AGE) globally, responsible for nearly 20% of all episodes of diarrheal disease. Currently, there are no approved vaccines available to prevent norovirus.
Vaxart received funding and support from the Gates Foundation to investigate whether vaccinating nursing mothers with its oral pill norovirus vaccine candidate protected their breastfeeding infants.
Heading Title
Description
Influenza
Vaxart’s Oral Vaccine: A Promising Alternative
to Traditional FluShots
The unmet medical need for influenza prevention remains significant despite the availability of seasonal flu vaccines. Current flu vaccines offer variable protection, with effectiveness ranging from 40% to 60% depending on the season and how well the vaccine matches circulating strains.
In a completed Phase 2 study, the efficacy of Vaxart’s oral pill vaccine compared favorably to market-leading Fluzone®.
Heading Title
Description
Human Papilloma Viruses (HPV)
Vaxart’s HPV Therapeutic Vaccine: A Promising Non-Invasive Treatment for Cervical Pre-cancers
Human papillomavirus (HPV) is a common virus that can cause cancers later in life. HPV 16 and HPV 18 are responsible for approximately 70% of cervical cancers and 50% of high-grade pre-cancerous cervical lesions.
Vaxart’s HPV therapeutic vaccine shows promise as a novel, non-invasive treatment for HPV-related cervical dysplasia.
Heading Title
Description




