
Our Science
Revolutionizing Immunizations with VAAST®
Vaxart has developed a unique modular vaccine platform called Vector-Adjuvant-Antigen Standardized Technology (VAAST).
Vaxart’s Proprietary VAAST Platform: Vector-Adjuvant-Antigen Standardized Technology (VAAST)
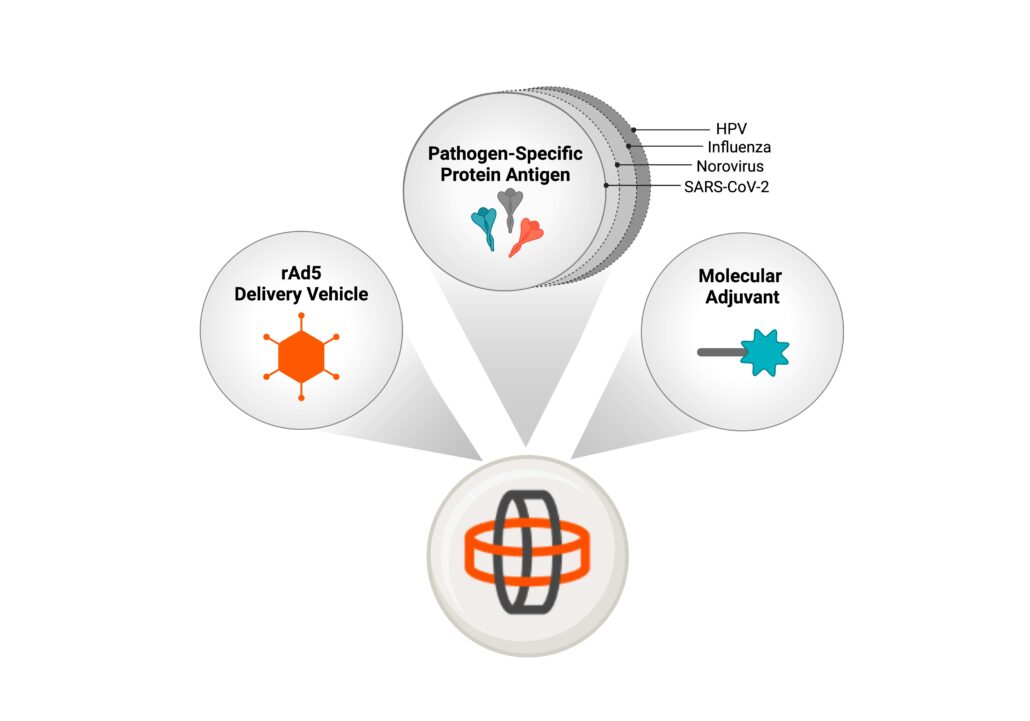
VAAST combines
three key components:
- Recombinant Adenovirus Type 5 (rAd5): non-replicating recombinant delivery vector
- Antigens: viral proteins that stimulate the immune system to recognize the targeted pathogen
- Molecular Adjuvant: enhances the immune response, making the vaccine even more effective
The benefit of the VAAST system allows protein antigens to be inserted easily into the nucleic acid-based vector to rapidly create vaccines designed to address both existing diseases as well as new and emerging threats.
Our Novel Oral Pill Vaccine Approach Triggers a First Line of Defense Against Viral Pathogens
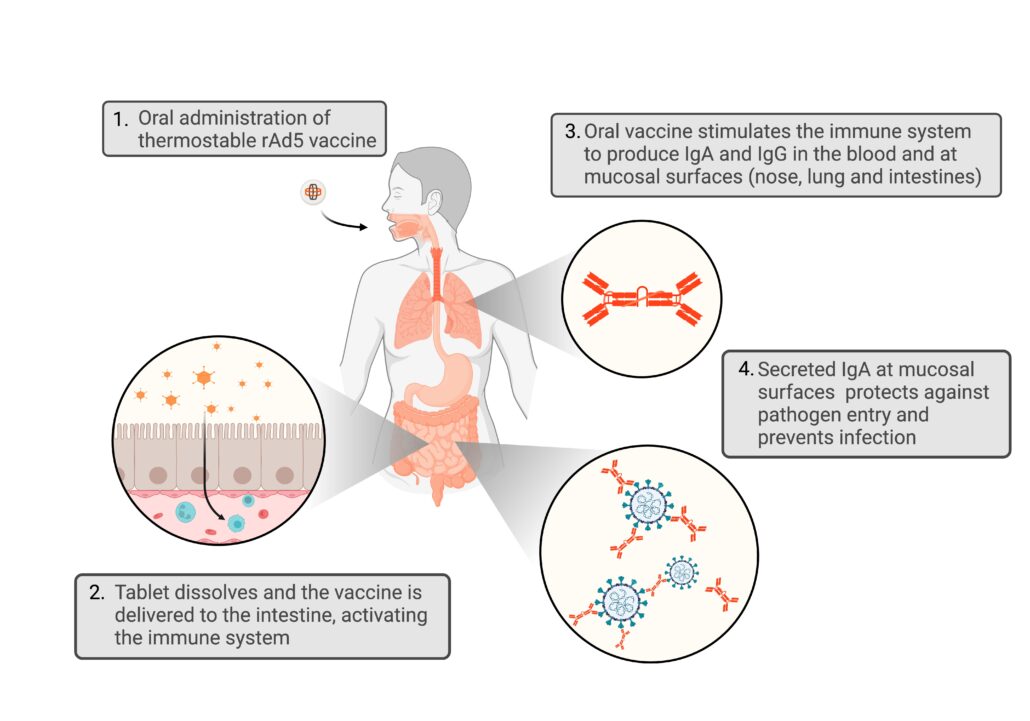

Our technology is purposefully designed to deliver the vaccine directly to the small intestine. The high concentration of lymphoid cells makes the small intestine well-suited for stimulating an immune response beyond serum antibodies. These other immune responses include local and distal mucosal barrier surface antibodies (IgA) and T-cell responses that could enhance protection against infections.
Our innovative oral immunization approach trains the immune system to recognize and destroy pathogens at key defense points – both at mucosal barrier surfaces like the airways and intestines (where pathogens first enter the body) as well as in the bloodstream. There has been a recent appreciation for the importance for mucosal immunity in protecting against respiratory and gastrointestinal infections. Vaxart’s oral delivery technology has demonstrated its approach can generate mucosal and systemic immune responses in multiple human studies.
The Aim of The Vaxart Oral Pill Vaccine Platform
Transform Global Health by Pioneering Oral Pill Vaccines That Provide Both Mucosal and Systemic Immune Responses Against Infectious Diseases
Our innovative oral pill vaccines stimulate both mucosal and systemic immune responses which may offer a unique advantage. Unlike traditional injectable vaccines, which primarily activate systemic immune responses, our oral pill vaccines engage the immune system at the mucosal level — the first line of defense against invading pathogens.
This dual-action approach may enhance protection and could provide a more comprehensive immune response.


Our oral pill vaccine platform has the potential to improve individual immunity. Additionally, because our vaccine is administered as a convenient oral pill, it may also offer broader public health benefits, setting us apart from conventional injectable and mRNA-based vaccine technologies.
By combining effectiveness with ease of administration, our approach paves the way for more accessible and adaptable solutions for global health challenges.
What Makes the Vaxart Platform Different?
-

Durable, Cross-reactive, and Mucosal Immunity
Our vaccine triggers both mucosal and systemic immune responses that are long-lasting and cross-reactive within virus families. The mucosal immune responses are both local to the intestinal delivery site and distal, potentially providing protection across multiple mucosal surfaces, including the nose, the mouth, and the intestines. Potent vaccine-induced antibodies were even induced in breast milk of lactating mothers, which could provide potential immunization benefits from mothers to their young children. Because mucosal surfaces are the first line of defense for multiple pathogens including COVID-19, influenza and norovirus, viruses that are known to undergo frequent mutation, a durable and broad immune response at the mucosa could provide enhanced protection against disease compared to traditional vaccines.
-

Convenient Oral Administration
Vaxart’s oral pill vaccine is designed for convenient, self-administration, eliminating the need for needles. Our oral pill vaccine could help overcome needle-related hesitancy and could reduce healthcare infrastructure traditionally needed to administer needle-based vaccines, thereby offering the promise to improve accessibility and public health.
-
State-of-the-art Recombinant Technology
Our oral pill vaccines eliminate injection site reactions, potentially leading to improved tolerability. Unlike some traditional immunizations, our vaccines do not contain live, replicating virus or animal products, which means they could offer an alternative to a broader range of individuals, including immunocompromised populations.
-
Thermostable
Vaxart’s oral pill vaccines are designed to maintain their efficacy without the need for refrigeration, unlike many injectable vaccines that require complex, cold chain storage. This increased stability can make distribution easier and more cost-effective, especially in areas with limited infrastructure.
-

Rapid Development
Our modular, scalable, and standardized approach to vaccine development supports the rapid development of various vaccines against established targets as well as against new and emerging pathogens.
-

Global Accessibility
A thermostable oral pill vaccine offers the potential for self-administration, empowering patients to manage their own immunizations. This easy-to-administer dosage form could significantly reduce healthcare costs, improve accessibility, and enhance distribution, particularly in remote or underserved areas.
Learn About Our Progress
Explore how our groundbreaking science is paving the way for a diverse pipeline of next-generation vaccines.
